Clues to the genetic factors underlying greater susceptibility to severe COVID-19 in men
Published: 2021-11-03
The COVID-19 pandemic has challenged healthcare world-wide for almost two years. Research has shown that there is significant inter-individual clinical variability in SARS-CoV-2, with the virus causing asymptomatic disease in some cases, whilst the disease is fatal in others. Identifying the risk factors for the development of more severe, or even fatal, COVID-19 cases has become increasingly important
The COVID Human Genetic Efforts consortium assembled an international cohort of patients to identify these risk factors. Specifically, to investigate the genetic and immunological factors that increase the risk of developing more severe forms of COVID-19. Previous results from the consortium have found that about 10% of critical cases of COVID-19 pneumonia are due to inborn errors of type I interferons (IFNs) or auto-antibodies to type I IFNs (Zhang et al., Science 2020; Bastard et al., Science 2020; Zhang et al., Med 2020). The consortium has also used these discoveries to develop therapeutic applications (Levy et al., J Clin Immunol 2021, de Prost et al., J Clin Immunol 2021; and Bastard et al., J Clin Immunol 2021).
The most prominent risk factor identified to date is increasing age - the risk of developing severe symptoms rises rapidly after 65 years of age (cf. Zhang et al. 2020). A number of studies have also indicated males are at greater risk than females (OR=1.5). Several research efforts have therefore focused their efforts on investigating possible X-linked risk factors.
In a recent Science Immunology publication, an international research collaboration led by researchers from The Rockefeller University, New York, USA (first author: Takai Asano, corresponding author: Jean-Laurent Casanova) examined whether non-synonymous variants in X-linked genes are a risk factor for developing COVID-19 pneumonia. MD Peter Bergman, researcher at Karolinska Institute, has led the clinical part of the study in Sweden. Prof Yenan Bryceson, Prof. Petter Brodin, Prof. Qiang Pan-Hammarström, and Prof. Lennart Hammarström, all also at Karolinska Institute, are also part of this collaboration.
Previous research has shown that autosomal inborn errors of type I IFN immunity, and autoantibodies against these cytokines, may be responsible for 1 in 10 of critical COVID-19 pneumonia cases. In this recent study, Asano and colleagues studied an international cohort of male patients (N=1202), aged between 6 months and 99 years (Average age = 52.9 years) that had experienced an unexplained severe, life-threatening case of COVID-19. The participants had no known inborn errors of TLR3- and IRF7-dependent type I IFN immunity, and no neutralizing auto-Abs against type I IFNs. The control group (N=331) comprised of males that experienced either an asymptomatic or mild case of COVID-19 infection. Whilst the age range of the control group was comparable to the age range of the experimental cohort, the average age in the control group was lower (around 39 years). The exomes (n = 1035) or genomes (n = 498) of both the patients and control individuals were sequenced and the researchers used the Genome Analysis Software Kit (GATK) best-practice pipeline to analyze the WES data.
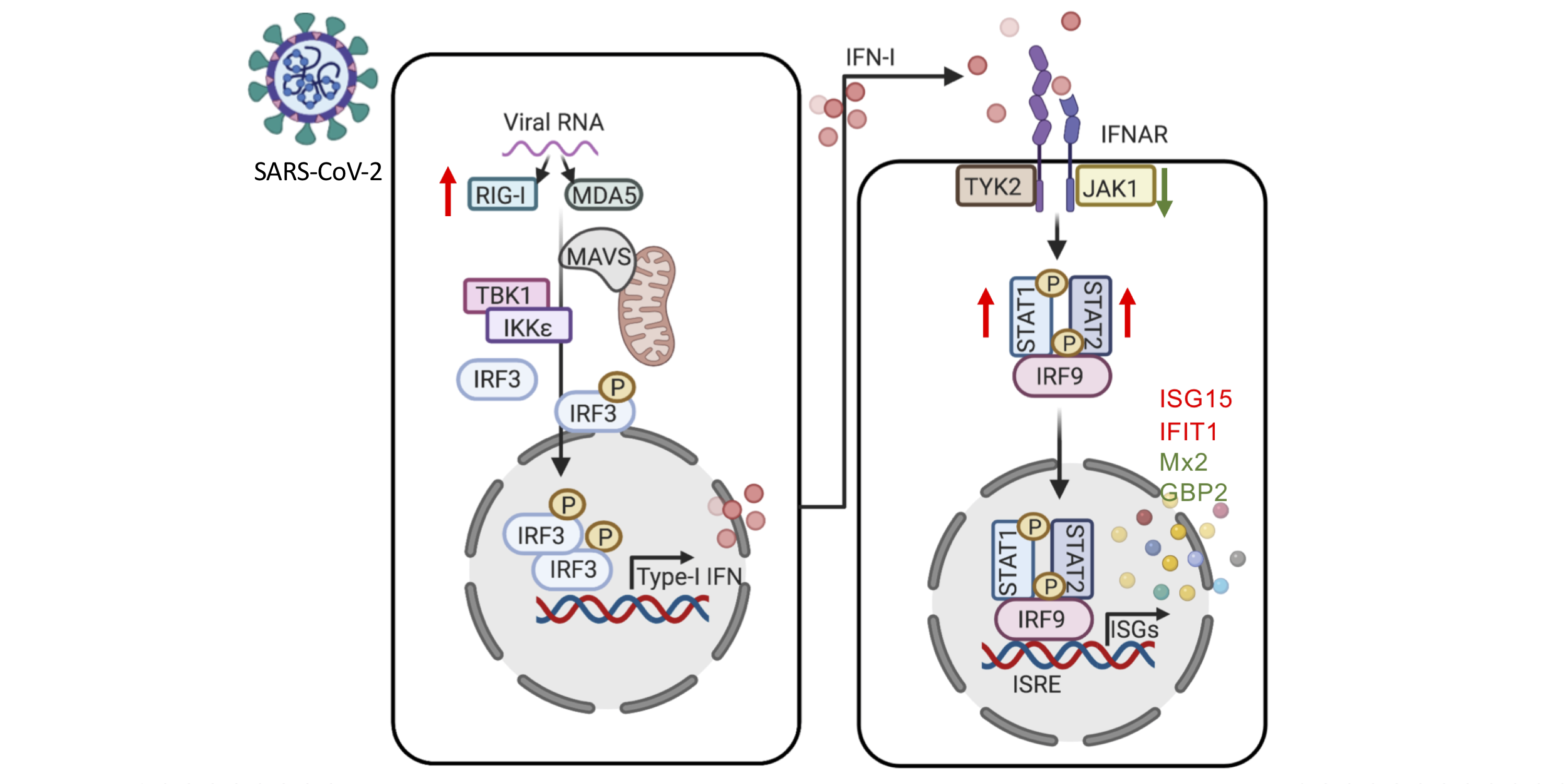
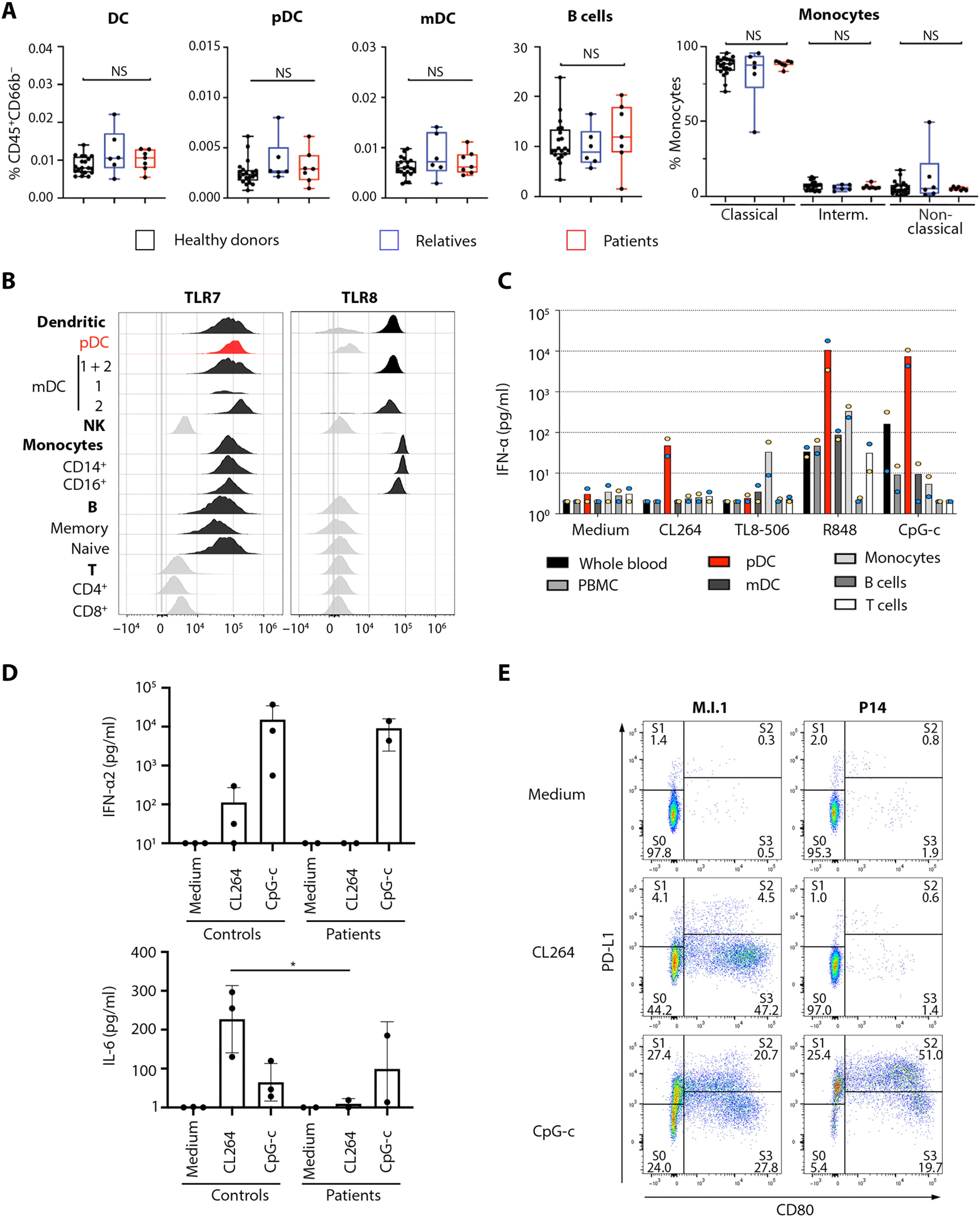
The researchers found an enrichment in very rare nonsynonymous TLR7 variants in 21 of the patients that experienced a severe, life-threatening case of COVID-19 infection. These 21 unrelated patients carried 20 different TLR7 alleles. In further biochemical analyses, the 20 TLR7 mutant proteins were expressed in human embryonic kidney (HEK) 293T cells. These latter analyses revealed that 16 of the males in the cohort exhibited very rare, biochemically deleterious X-linked Toll-like receptor 7 (TLR7) variants that resulted in TLR7 deficiency. Most of the males with the TLR7 deficiency were younger than 60 years (average age= 36.7, age range 7-71 years). In comparison, none of the controls carried such TLR7 variants.
TLR7 is a receptor on a cell in the immune system that stimulates the blood plasmacytoid dendritic cells (pDCs), these pDCs are the primary producers of type I interferon (IFN-I). This is an important part of the defense against viruses, such as the new SARS-CoV-2. Asano and colleagues showed that these pathways were changed in the 16 male patients with rare TLR7 variants (heterozygous or in some cases homozygous). The researchers found that the blood B cell lines and myeloid cell subsets from the 16 male patients with TLR7 deficiency did not respond to TLR7 stimulation, a phenotype rescued by wild-type TLR7. Additionally, the 16 male patients’ blood pDCs produced lower levels of type I IFNs in response to SARS-CoV-2.
“Interestingly, two Swedish patients were included in the study. They had a unique mutation in the TLR7 gene, which was deleterious. This was shown by stimulating plasmacytoid dendritic cells from these patients with a TLR7-specific ligand, which showed a significantly reduced or absent interferon alpha response compared to controls. The patients were brothers, previously healthy and had severe COVID-19, which required treatment in the ICU-department. This finding could be very important for other male relatives, who could be carriers. However, it seems that vaccination against COVID-19 is an efficient preventive measure also in these patients,” says Peter Bergman, researcher at Karolinska Institutet.
In summary, Asano et al. identified an X-linked recessive TLR7 deficiency that is a highly penetrant genetic etiology of critical COVID-19 pneumonia that affects about 1 in 50 male patients below 60 years of age. They conclude that TLR7 and pDCs are important part of the defence against SARS-CoV-2 in the respiratory tract.
The researchers have deposited the RNA-seq data for this study in the Gene Expression Omnibus (GEO) database under accession number GSE181787. GEO is a public functional genomics data repository supporting MIAME-compliant data submissions, it accepts botharray- and sequence-based data. Additional source data needed to evaluate the conclusions in the paper are present in the paper, and the Supplementary Materials in the fulltext version of the paper. The protocol for the paper was shared in Bio-protocol.
The researchers that conducted this study were supported by a large number of international grants, including the Howard Hughes Medical Institute, Rockefeller University, the St. Giles Foundation, the National Institute of Health (NIH), the National Center for Advancing Translational Sciences (NCATS), NIH Clinical and Translational Science Award program, INSERM etc. Peter Bergman also received support from the Center for Medical Innovation (CIMED), the Swedish Medical Research Council and the Stockholm County Council (ALF-project). Part of this work was generated within the European Reference Network for rare primary immunodeficiency, autoinflammatory and autoimmune diseases (RITA).
Data
- RNA-seq data: Available at GEO, accession number GSE181787
- Protocols from the study: Available at Bio-protocol
Article
DOI: 10.1126/sciimmunol.abl4348
Takaki Asano, Bertrand Boisson, Fanny Onodi, Aniela Matuozzo, Marcela Moncada-Velez, Majistor Raj Luxman Maglorius Renkilaraj, Peng Zhang, Laurent Meertens, Alexandre Bolze, […], Jean-laurent Casanova. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19, Science Immunology 6 (62) (2021).